Blogs

Nancy Myers to speak at 4th Agentic AI Innovation Summit at #JPM26 conference.
Catalyst’s Nancy Myers is looking forward to speaking on a panel at the 4th Agentic AI Innovation Summit on January 12 at 11 am. She will speak alongside Vada A. Perkins, Andrew Robertson, Jimita Parekh, and moderator Brad Pryde. If you are attending, we would love to chat – reach out to us on LinkedIn!
Nancy Myers Speaks on Better Leveraging Existing Knowledge During OTP Public Listening Meeting
Catalyst CEO, Nancy Myers, had the opportunity to address CBER leadership during OTP’s September 18th public listening meeting titled “Leveraging Knowledge for Facilitating the Development and Review of Cell and Gene Therapies.” Though a PDUFA VII commitment and a required action, meetings like this one provide a critical space for industry and the Agency to share ideas and deliver better outcomes for patients.
.jpg)
Plan for Consolidating FDA’s Adverse Event Reporting Systems: Good Idea but Easier Said than Done
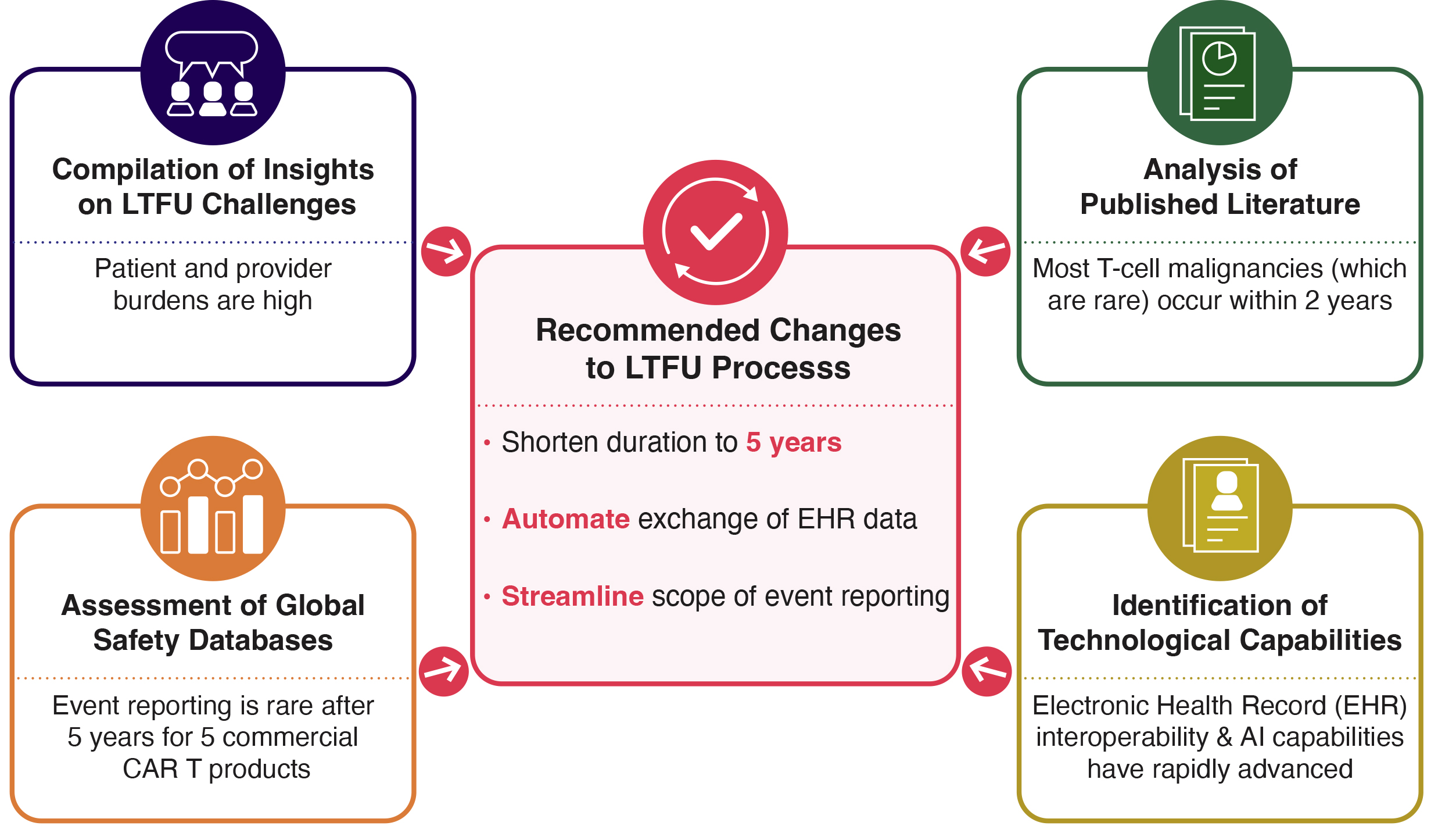
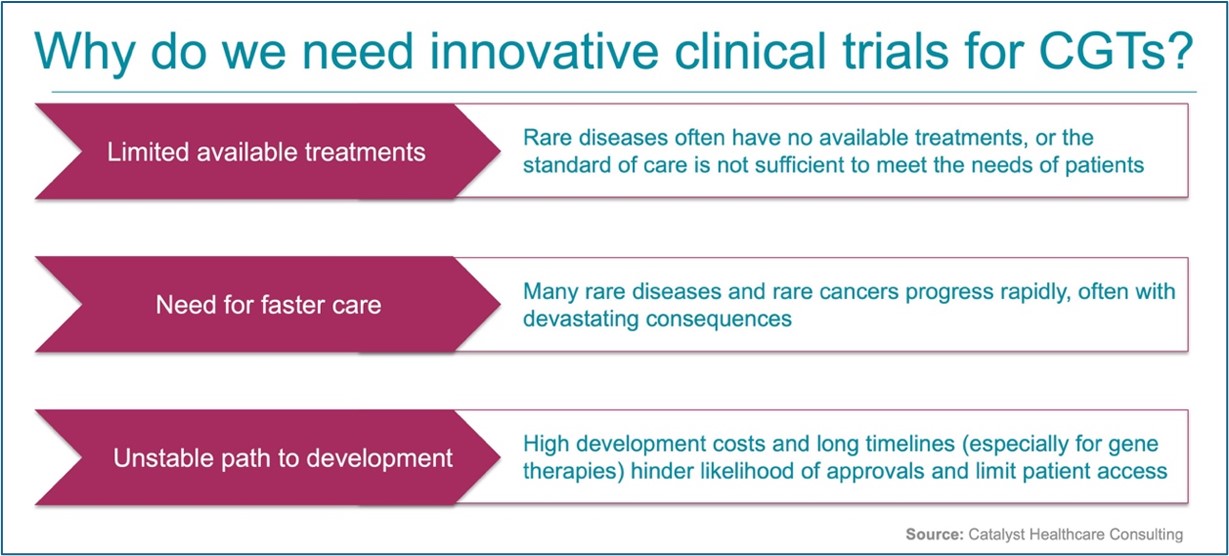
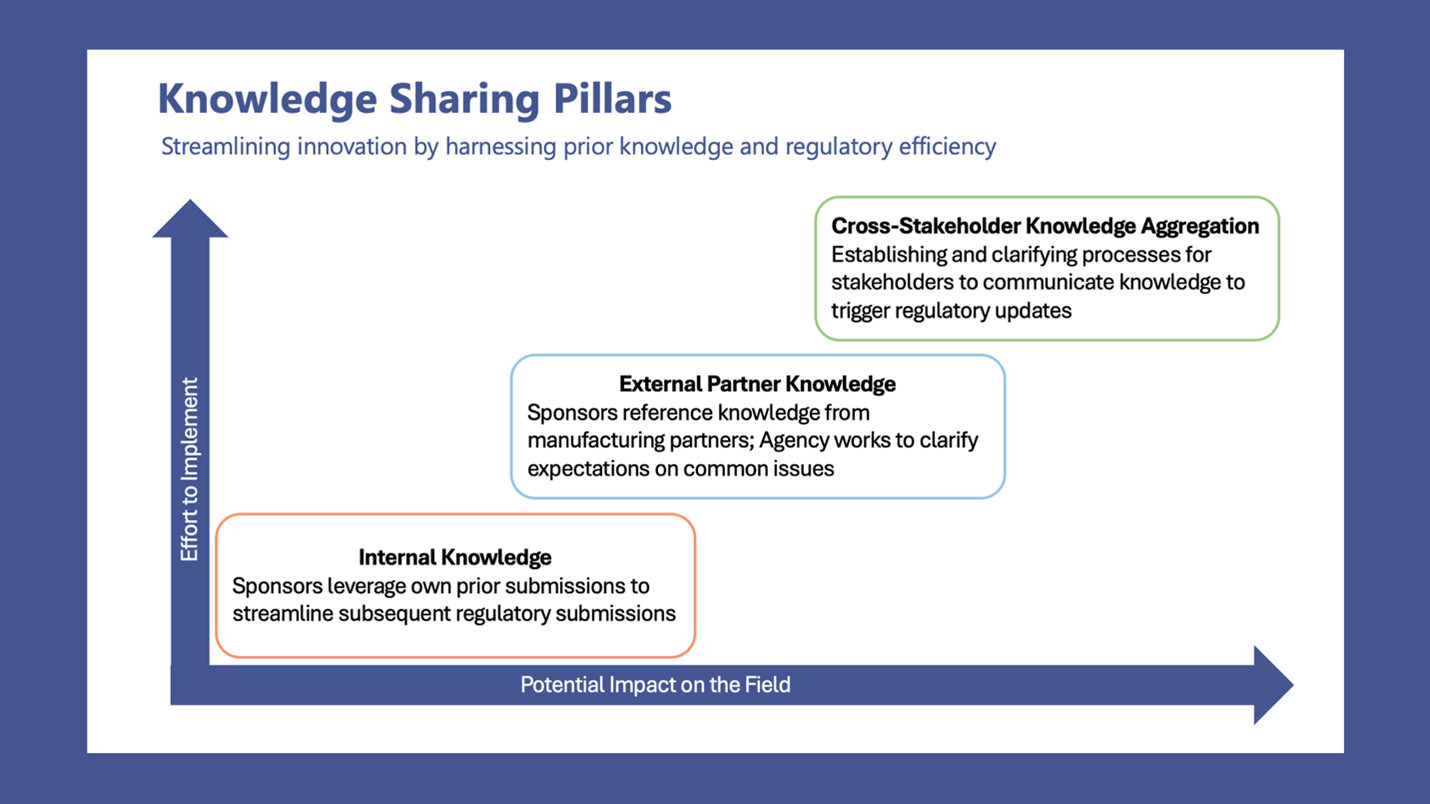
NEW WHITE PAPER: Data-Informed Optimization of CAR T-Cell Therapy Long-Term Follow-Up (Preprint)
Multistakeholder collaboration, led by Catalyst Healthcare Consulting, to Recommend Data-Informed Optimization of CAR T-Cell Therapy Long-Term Follow-Up (preprint).

Catalyst to attend FDLI 2025 Annual Conference
If you are attending the FDLI conference, we would love to chat - reach out to us on LinkedIn!

Catalyst advances women’s health in new LinkedIn article
On Tuesday, Catalyst’s Taryn Serman and Nancy Myers, together with industry leaders, published the following article regarding women’s health on LinkedIn.

Government Shutdown 101: What to Expect
March 14th is approaching fast, and it increasingly looks like politicians are driving us toward another government shutdown.

Expected FDA Impact of a Government Shutdown
Critically, the critical public health mission and user fees are the buffer for FDA when a shutdown occurs. Below, we will delve into how a shutdown may look for the FDA under the Trump administration.

Califf: FDA will be a ‘more activist’ agency
This article describes an interview co-moderated by Catalyst CEO Nancy Myers, along with Tom Kraus, VP- Government Relations, American Society for Health-System Pharmacists. The webinar interview was sponsored by the Alliance for a Stronger FDA.