Nancy Myers Speaks on Better Leveraging Existing Knowledge During OTP Public Listening Meeting
September 25, 2025
Catalyst CEO, Nancy Myers, had the opportunity to address CBER leadership during OTP’s September 18thpublic listening meeting titled “Leveraging Knowledge for Facilitating the Development and Review of Cell and Gene Therapies.” Though a PDUFA VII commitment and a required action, meetings like this one provide a critical space for industry and the Agency to share ideas and deliver better outcomes for patients.
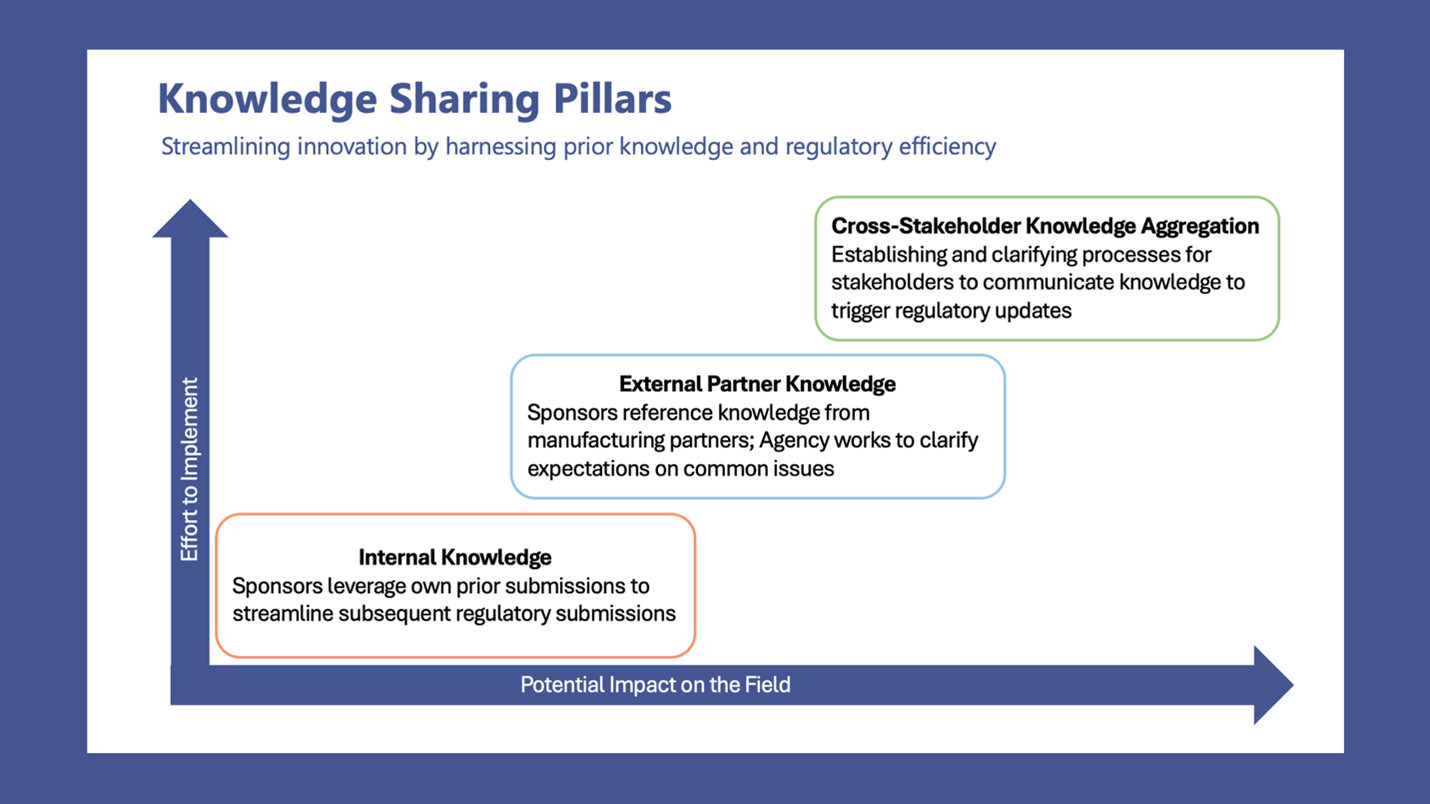
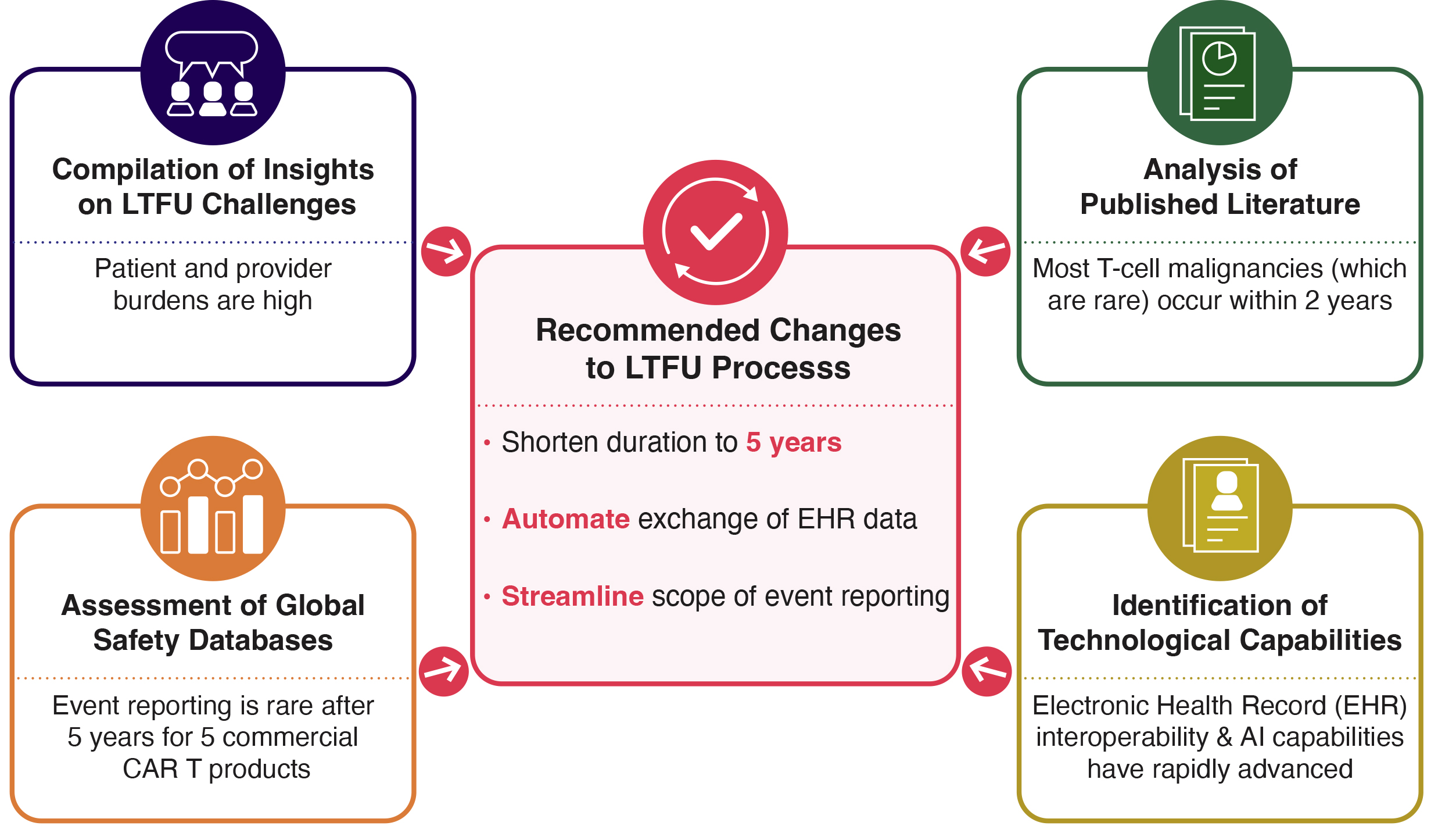
Speaking from her capacity as a consultant who sees across multiple pipelines, Nancy introduced a framework for CBER to better leverage existing knowledge at three levels: 1) within a sponsor, 2) across external partners, and 3) throughout the broader health ecosystem. Her suggestions included:
Nancy advocated for these steps as examples of actions that could be taken to help sponsors and the Agency better harness existing evidence. This is particularly relevant currently as the FDA’s focuses on individualized and rare disease therapies accelerators. By promoting policies which prioritize the smart, appropriate leveraging of data and recommitting to regulatory clarity, the Agency has the opportunity to expand patient access, streamline regulatory decision-making, encourage the development of novel therapies, and reduce development costs.
If you would like access to Nancy’s full slide show, please click the link below!



.jpg)

