Catalyst, in collaboration with the Emily Whitehead Foundation, publishes Amplifying the Voice of CAR T-Cell Therapy Patients and Caregivers
December 16, 2024

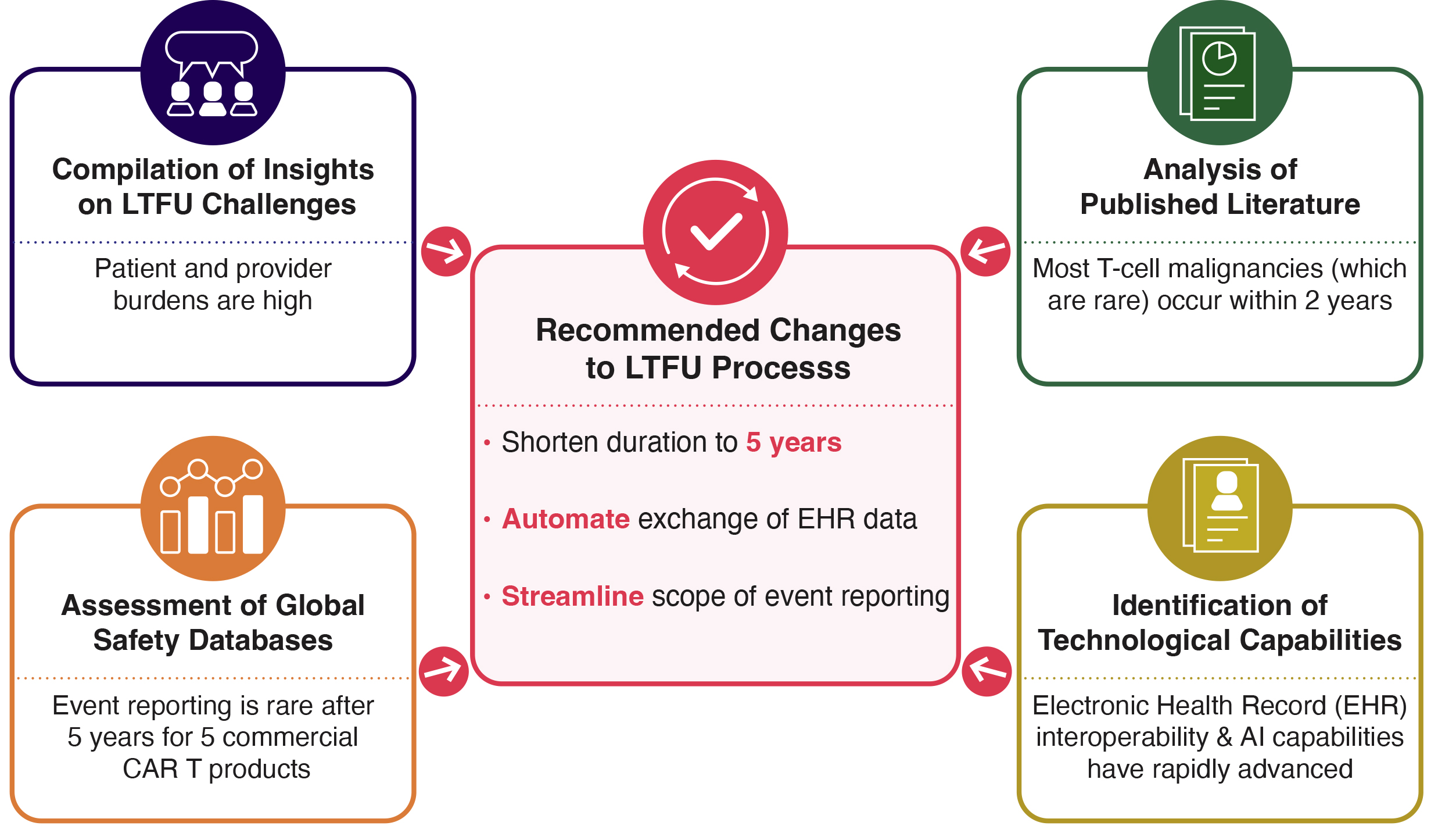
Understanding the patient experience with CAR-T long-term follow-up (LTFU) is essential for building a sustainable model. Catalyst Healthcare Consulting, in partnership with the Emily Whitehead Foundation, surveyed nearly 100 patients and caregivers to capture their perspectives.
The findings are outlined in the whitepaper Amplifying the Voice of CAR T-Cell Therapy Patients and Caregivers, which highlights the urgent need for patient-centered LTFU processes — an increasingly critical focus as more CAR T-cell therapies receive FDA approval and reach a growing number of patients



.jpg)

