Catalyst Driving Discussions to Accelerate C> Trials: DIA 2025
May 15, 2025
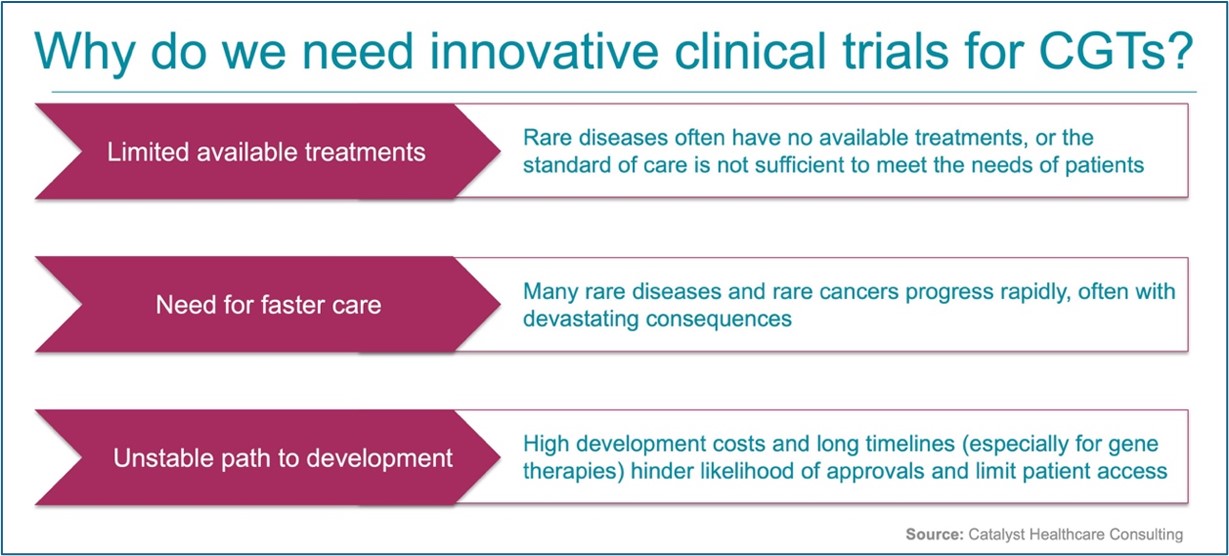
At the recent DIA 2025 Global Annual Meeting, Catalyst’s CEO Nancy Myers led a dynamic panel of distinguished experts who discussed how innovative Master Protocol trial designs—particularly umbrella trials—can accelerate the path to “platformize” cell and gene therapies (CGTs) to meet the needs of the rare disease patient community. Joining us were Dr. Kiran Musunuru (Professor of Medicine at University of Pennsylvania), a leading voice in Cell & Gene Therapy and a pioneer in base editing; Dr. PJ Brooks (Deputy Director of the Division of Rare Diseases Research Innovation at NIH), a distinguished thought leader in the generation of streamlined GT development platforms; and Dr. Jeff Allen (President and CEO of Friends of Cancer Research), an esteemed oncology expert and advocate.
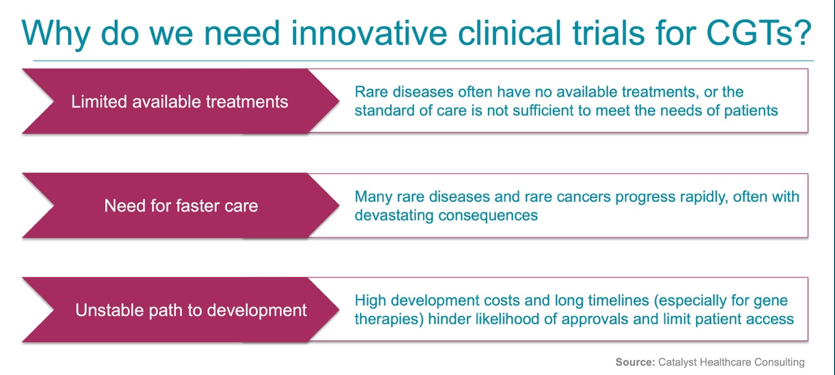
Throughout the discussion, Nancy and panelists discussed lessons learned from the oncology field, applications of umbrella and basket trials, and opportunities that are emerging under the new FDA administration. The conversation spotlighted umbrella trials, which evaluate multiple targeted therapies for a single disease under one Master Protocol, enabling efficiencies via shared infrastructure and adaptive trial arms to speed results and approvals. These designs lay the groundwork for platform therapies, which reuse key components across multiple treatments, allowing existing data to be reused to support the rapid development and approval of new therapies.
Although the FDA’s Platform Technology Designation draft guidance permits data sharing across therapies, it requires the first therapy of the platform to be fully FDA-approved prior to data sharing. Many in the CGT field are eager to bypass this prerequisite, especially in the GT space. GTs often use the same delivery vector (e.g., viral or lipid nanoparticles) to carry different healthy genes or gene-editing instructions (i.e., guide RNAs, “gRNAs”). Platformizing GT vectors could mean swapping in new healthy genes or editing instructions without the need for re-evaluation and re-validation of vector safety—dramatically reducing timelines for rare disease treatments and offering options to those with life-threatening rare diseases.
Panelist Dr. Musunuru’s ground-breaking work exemplifies this game-changing potential: his FDA collaboration led to the rapid, life-saving treatment of “Baby KJ” for CPS-1 deficiency and paved the way for FDA to endorse a novel umbrella/platform trial framework to fast-track similar therapies for other rare inherited metabolic disorders—a transformative step for the future of CGTs.

It was a pleasure connecting with everyone who joined our DIA panel, and we’re eager to see the continued progress in advancing CGTs through innovative platform trial approaches.



.jpg)

