May 15, 2025

Catalyst is pleased to attend the FDLI 2025 Annual Conference, where leaders from across the healthcare environment come together to discuss the most pressing issues in food and drug law.
We’re especially looking forward to hearing Dr. Ellen Sigal’s remarks as she receives the prestigious Dr. Harvey W. Wiley FDAAA Award – one of FDLI’s highest honors. Named after the first commissioner of the US Food and Drug Administration, the award recognizes individuals who have made outstanding contributions to promoting public health and advancing FDA’s regulatory mission.
As Chairperson and founder of Friends of Cancer Research, Dr. Sigal has been a driving force behind transformative policy and regulatory initiatives that accelerate access to life-saving treatments. Her leadership spans influential roles at NIH, PCORI, the Reagan-Udall Foundation, and the Cancer Moonshot initiative, among many others.
Congratulations, Dr. Sigal!
If you are attending the FDLI conference, we would love to chat - reach out to us on LinkedIn!
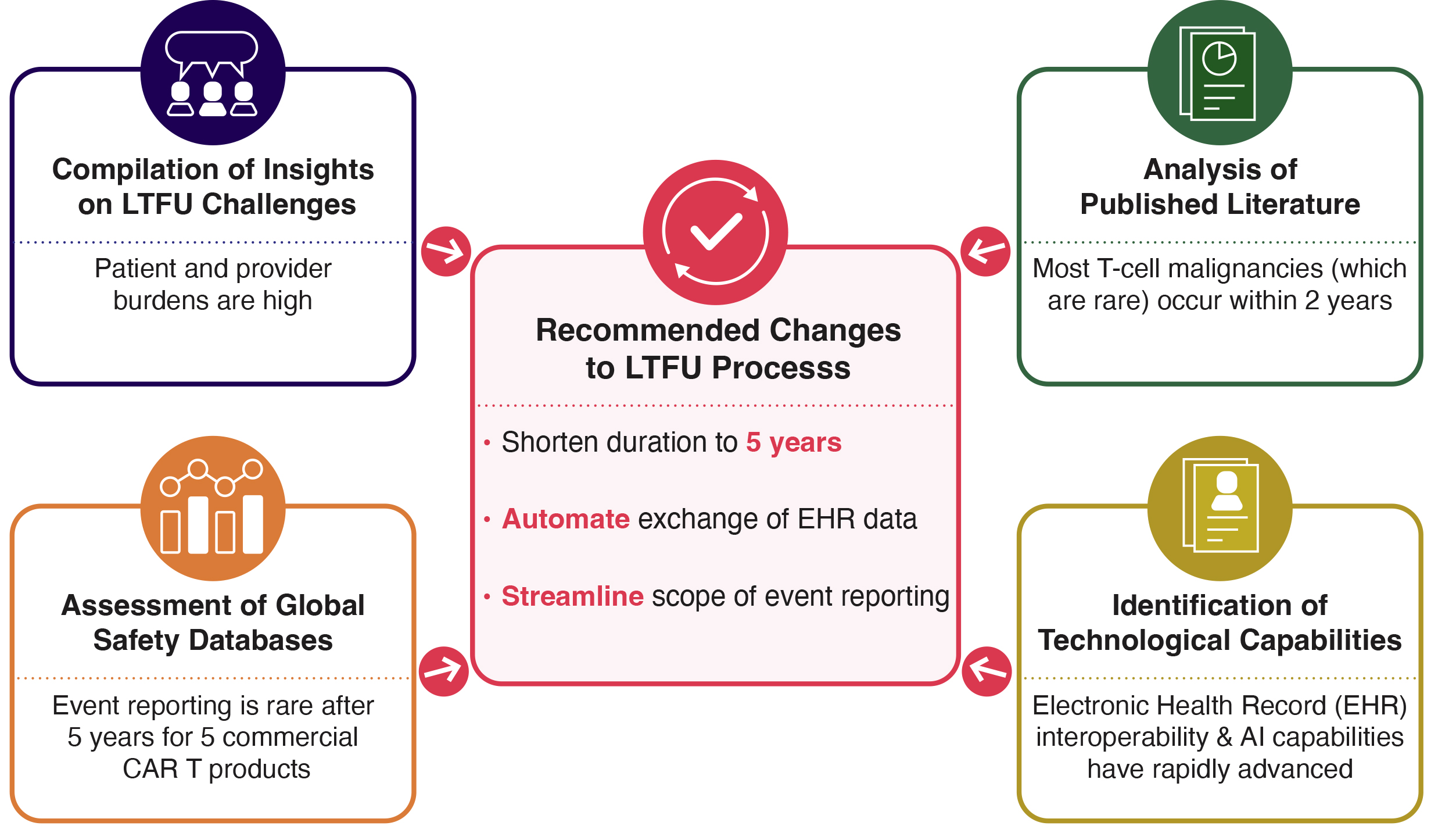



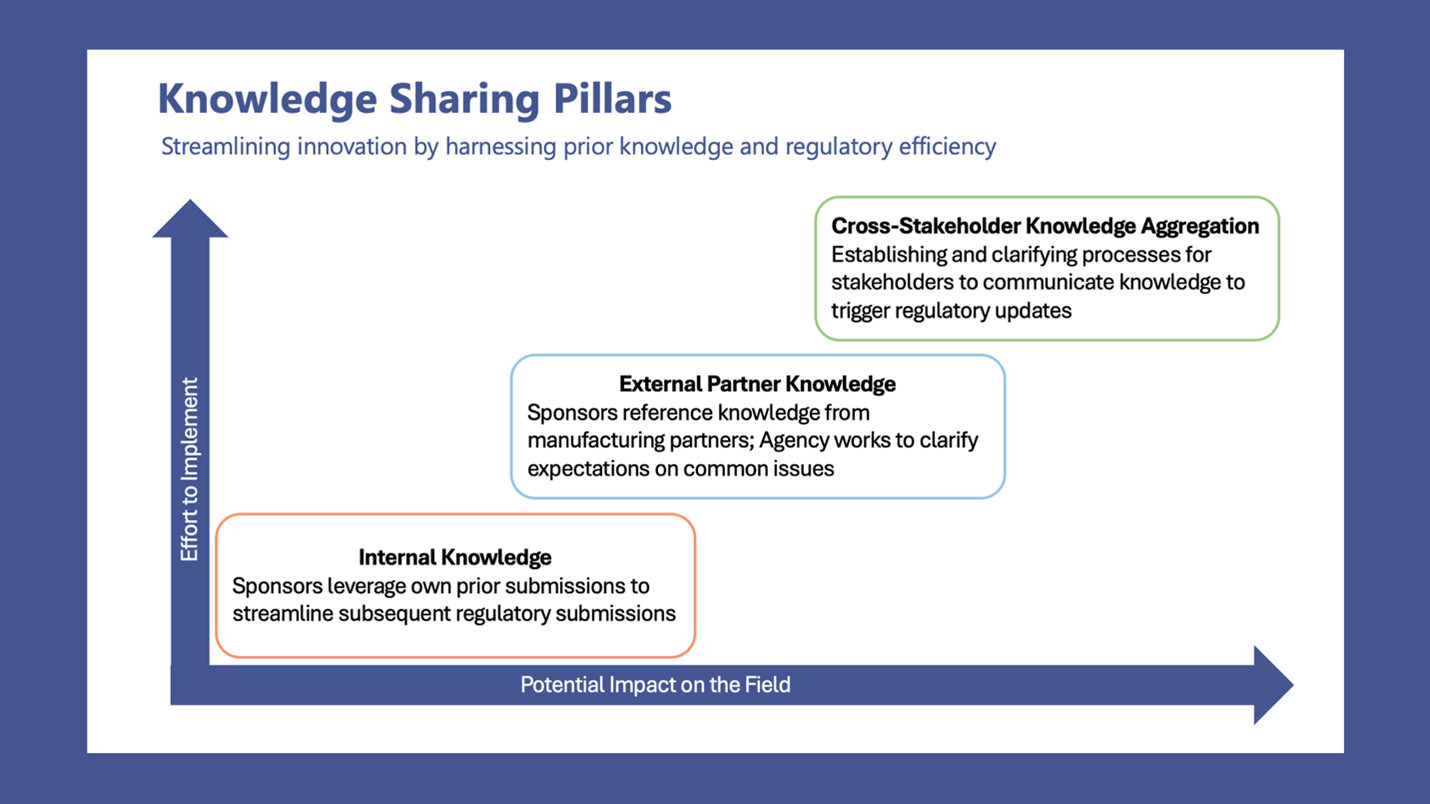
.jpg)

