Multistakeholder collaboration, led by Catalyst Healthcare Consulting, to recommend Data-Informed Optimization of CAR T-Cell Therapy Long-Term Follow-Up (preprint).
August 6, 2025
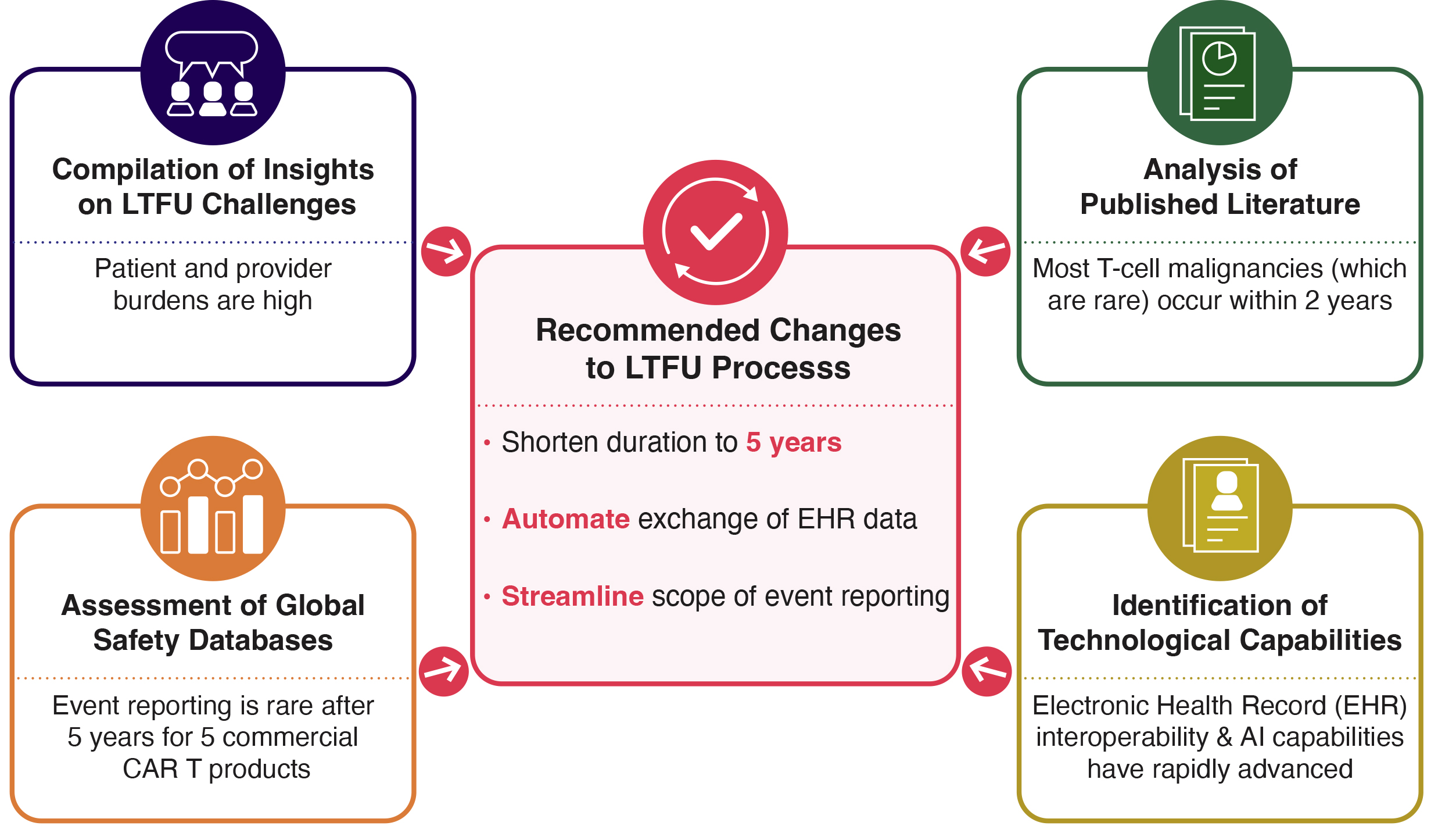
Given the field’s extensive experience with CART-cell therapies, it is worth asking whether 15 years of long-term follow-up(LTFU), carried out through complex processes, remains scientifically necessary or operationally realistic for patients and providers. To assess this question, Catalyst convened representatives from patient advocacy groups, academia, industry, and government. The group's resulting article (preprint linked) recommends reducing LTFU requirements to five years in both the clinical trial and commercial settings, backed by newly aggregated primary data and the literature on the timing of adverse events. Additionally, the authors propose a streamlined process that leverages technological advancements to automate the transfer of more focused safety data from electronic health records (EHRs) into a third-party database.
We thank our partners from the following organizations for their contributions to this article: Emily Whitehead Foundation, Friends ofCancer Research, Leukemia & Lymphoma Society, University of Pennsylvania, Kite Pharma, Bristol Myers Squibb, Johnson& Johnson, Novartis Pharmaceuticals, National Institutes of Health, A2 Biotherapeutics, and Artiva Biotherapeutics.






